Skylocalm 16mg pk100

MiPet Skylocalm 16mg film (box of 100) coated tablets for Dogs, for the treatment of pruritis or atopic dermatitis
MiPet Skylocalm (box of 100) 16mg tablets for Dogs are for the treatment of pruritis associated with allergic dermatitis in dogs, or for the treatment of clinical manifestations of atopic dermatitis in dogs.
Each film coated tablet contains 16 mg of oclacitinib (as oclacitinib maleate) for full list of excipients see product ingredients.
For Oral Use
Dosage and treatment schedule: The recommended initial dose is 0.4 to 0.6 mg oclacitinib/kg bodyweight, administered orally, twice daily for up to 14 days.
For maintenance therapy, the same dose (0.4 to 0.6 mg oclacitinib/kg bodyweight) should then be administered only once a day. The requirement for long-term maintenance therapy should be based on an individual benefit-risk assessment.
These tablets can be administered with or without food.
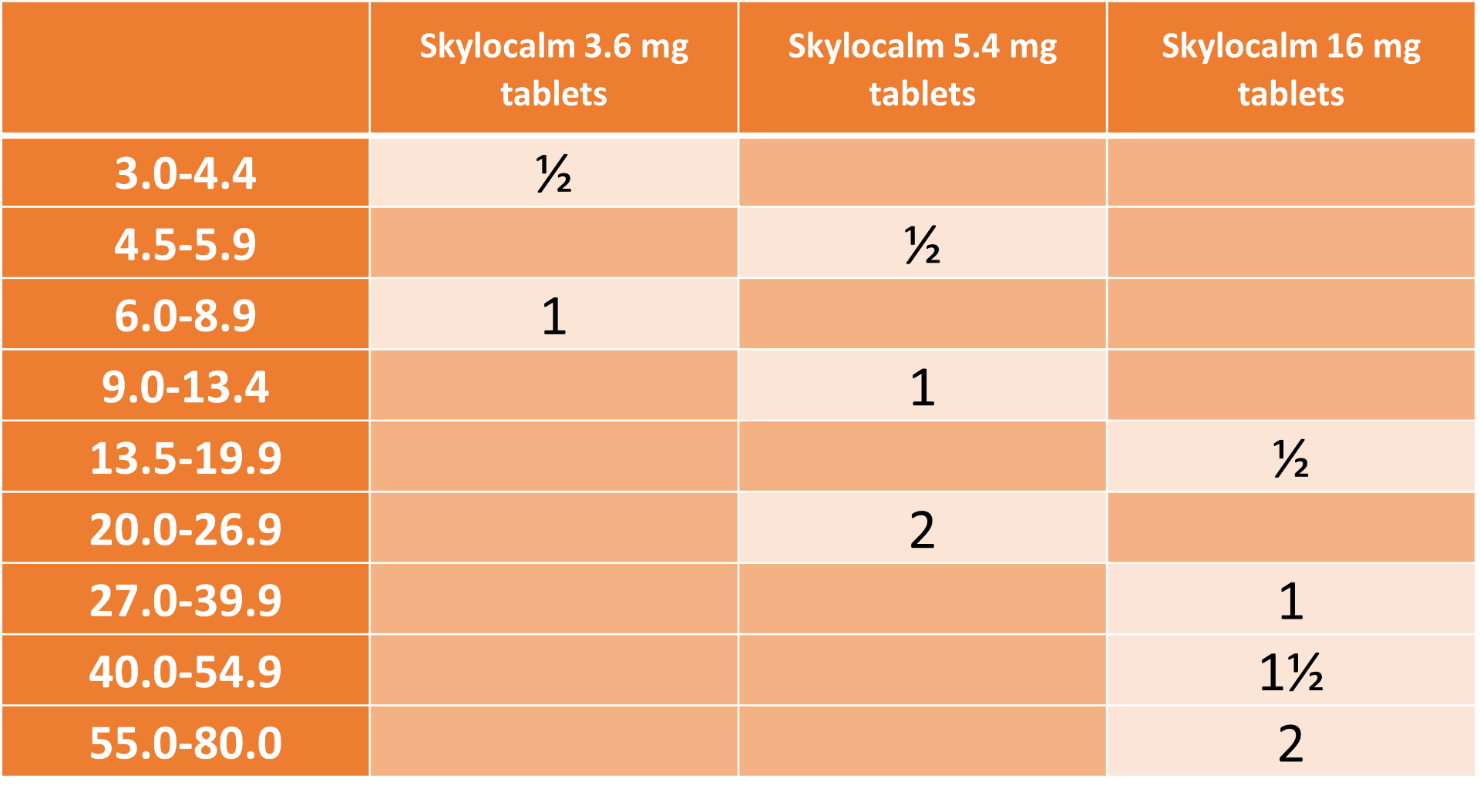
The dosing table below shows the number of tablets required. The tablets are breakable along the score line.
Active substance:
Each film-coated tablet contains 5.4 mg oclacitinib (as oclacitinib maleate)
List of excipients
Tablet core:
Cellulose, microcrystalline
Lactose monohydrate
Magnesium stearate
Sodium starch glycolate
Tablet coating:
Lactose monohydrate
Hypromellose (E464)
Titanium dioxide (E171)
Macrogol 400 (E1521)
Shelf life
Shelf life of the veterinary medicinal product as packaged for sale: 2 years. Any remaining half tablets should be discarded after 3 days.
Special precautions for storage
Store below 25°C. Any remaining half tablet should be placed back in the opened blister and stored in the original cardboard carton (for a maximum of 3 days).
Special precautions for use
i) Special precautions for use in animals
Oclacitinib modulates the immune system and may increase susceptibility to infection and exacerbate neoplastic conditions. Dogs receiving Skylocalm tablets should therefore be monitored for the development of infections and neoplasia.
When treating pruritus associated with allergic dermatitis with oclacitinib, investigate and treat any underlying causes (e.g. flea allergic dermatitis, contact dermatitis, food hypersensitivity). Furthermore, in cases of allergic dermatitis and atopic dermatitis, it is recommended to investigate and treat complicating factors, such as bacterial, fungal or parasitic infections/infestations (e.g. flea and mange).
Given the potential for effects on certain clinicopathological parameters (see section 4.6 of the SPC), periodic monitoring with complete blood counts and serum biochemistry is recommended when dogs are on long-term treatment.
ii) Special precautions to be taken by the person administering the veterinary medicinal product to animals
Wash hands after administration. In case of accidental ingestion, seek medical advice immediately and show the package leaflet or the label to the physician.
iii) Other precautions
None.

